Basically titration is a chemical method to determine the concentration of solution. The trick is to react with a solution in a certain volume with another solution with a known concentration of the substance. This known solution is called a frozen solution. While the purpose of the titration itself is to find out the pH level of a chemical. The end point is when there is a color change in the indicator.
This titration measurement usually uses several special tools, including burette, statif, erlenmeyer tube, suction rubber, watch glass, drop pipette, flask and volume pipette. One of the requirements for titration to run well, among others, is characterized by a rapid reaction, even using a catalyst to accelerate the reaction. Next, the reaction is simple and the stoichiometric equation is clear. Then there are no side reactions that can affect the main reaction. Then, what are the types of titration and how do you do the titration? Here's the review that you need to know.
Type of Titration
Based on the type, the titration is divided into four types. The three types are redox titration, complexation titration, and acid base titration and argentometry. For more details about the three types of titration you can refer to the following review.
Redox Titration
For the type of redox titration is the type of titration that processes with a redox reaction. Redox in titration is still divided into three. Namely that uses I2 and is an indirect titration. This is because the reacting I2 is still made with the previous redox reaction. While for the second type is iodometric titration which is used directly in I2 and can be called a direct reaction. The third redox type is permanganometry where the reaction utilizes Mn2 + ions.
Complexation Titration
This titration of complexation types is actually titration which uses complexation reactions and complex ion formation. Its use is usually to analyze metal content. If you want to do type titration there are several things to consider. This is more because the formation of complex ions is very specific in certain conditions.
Acid-Base Titration
The third type of titration is acid-base titration. Actually this titration refers more to quantitative analysis methods based on acid-base reactions. The indicators used are usually those that can profilize changes in color in a certain pH.
Argentometry Titration
This last type is argentometry titration. This titration is a titration commonly used for depositional reactions. Based on the principle, argentometry titration regarding solubility and also the resulting constant of the reacting reactor. Methods for titration Argentometry is divided into Mohr method, Volhard method, and Fajans method.
How to Do Titration
There are 2 ways to titrate: Manual and Automatic. The standard method used for manual titration in petroleum products and lubricants is ASTM D974. While the method used for automatic potentiometric titration of petroleum products, lubricants, and biodiesel is ASTM D664.
In doing a manual titration, there are a number of things that you need to prepare. Especially the equipment used such as burette, stative, and clamps, and erlenmeyer. Do not forget to also prepare a standard solution whose concentration can be known and this solution is placed on the burette. This solution is called the titration solution and its concentration must be known.
For the solution it is placed in a titration flask and hereinafter referred to as the solution titrated and the volume must be known. The titrated solution can then be dropped on an acid-base indicator. The pentetesan process can be stopped when the end point of the titration is reached. When all substances have finished reacting, the indicator's solution will change color.
To more easily learn how to titrate, try to consider the following steps.
Step 1:
The solution to be dropped is put into the burette (long scale pipe). The solution in the burette is called titration.
Step 2:
The solution to be titrated is put into erlenmeyer by measuring its volume first using the goiter pipette.
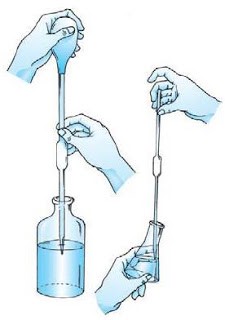
Image 1. Measuring the volume of solution using a goiter pipette.
Step 3:
Give a few drops of indicator to the solution titrated (in erlenmeyer) using a dropper pipette. The indicator used is the color change around the equivalent point.
Step 4:
The process of titration, ie the solution in the burette is dripped slowly through the faucet into the erlenmeyer. Erlenmeyer shakes so that the titration solution can dissolve with a solution that is in erlenmeyer. The addition of the titration solution into Erlenmeyer was stopped when there was a color change in Erlenmeyer. This color change indicates that the titration end point has been reached (equivalent point).
Step 5:
Record the volume needed for the titration solution by looking at the volume that is reduced in the burette after the titration process is carried out.
Steps to Manually Titrate
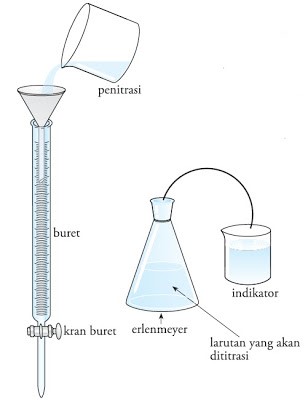
Image 2. Steps to do a manual titration.
Whereas the Potentiometric Automatic Titration process can be carried out with the help of an appropriate Electrode Indicator and comparative Electrode. The titration curve is obtained by drawing a potential graph of the pentiter volume added until the end point of the titration is reached. This method is more accurate for analyzing murky samples and low Acid Number values ??that are difficult to analyze using the manual titration method using a color change indicator.
The Hyprowira Adhitama Laboratory provides water content analysis services as well as the Mettler Toledo Potentiometric automatic titrator.





